The cannabinoid system consists of cannabinoids and cannabinoid receptors. cannabinoids can be subdivided into endocannabinoids, (produced by your own body, such as Anandamide), plant cannabinoids (produced by cannabis plants, such as THC) and synthetic cannabinoids (produced by industry, such as Marinol).
cannabinoids are lipid/fatty compounds, which are very poorly solvable in water but can easily migrate through fat/lipids such as cell membranes.
cannabinoid receptors are a diverse group of proteins that bind cannabinoids and convey their message. Most cannabinoid receptors are G protein-coupled receptors (see GPCR introduction), which means that the effect of their activation is highly variable and can be modulated in many ways.
In this section we will try to introduce each component of the cannabinoid system in a way that is both easy to understand and scientifically correct. We will give a brief general description of its characteristics, followed by a more complete scientific description of its synthesis, degradation, distribution and interactions and reference material.
Each entry is fully hyperlinked to their relevant partners so clicking through this section should give a fairly intuitive grasp of the web of interactions within the cannabinoid system. This forms the background against which the therapeutic properties of cannabinoids are discussed in a whole range of disorders.
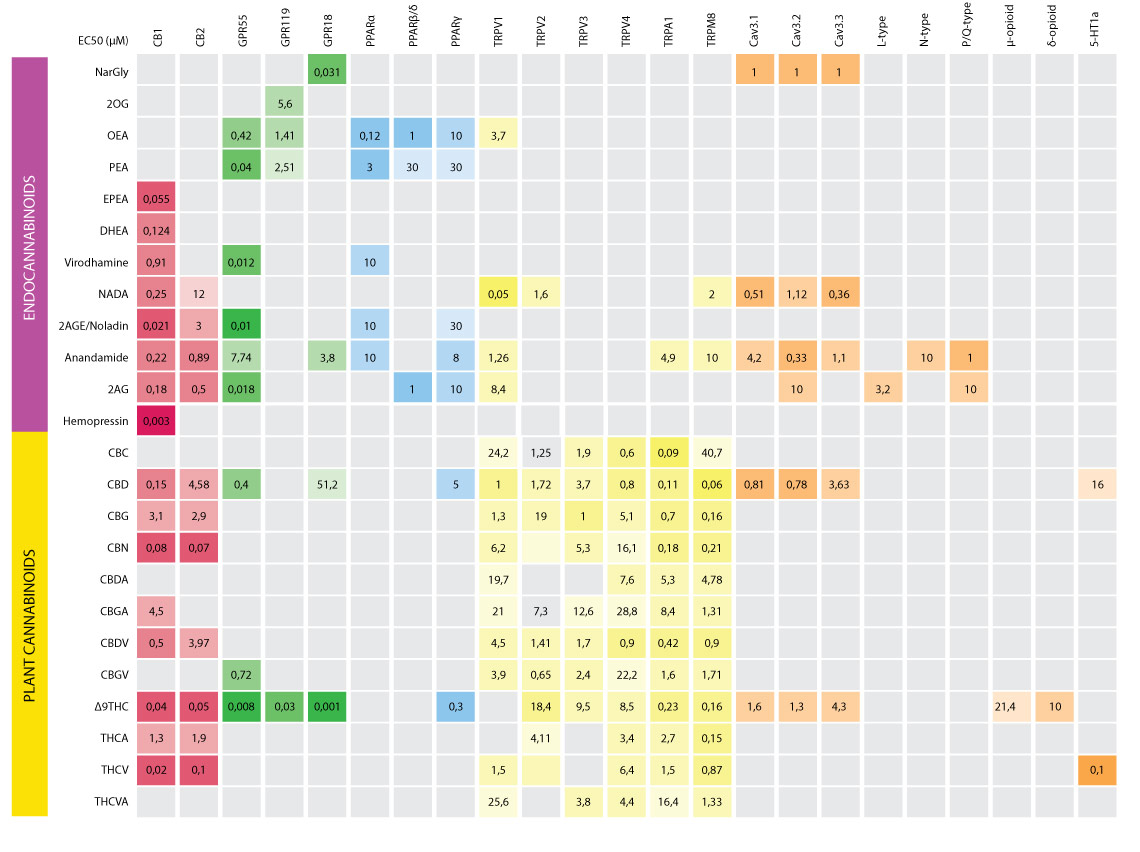
Literature:
Anavi-Goffer, S., Baillie, G., Irving, A.J., Gertsch, J., Greig, I.R., Pertwee, R.G., and Ross, R.A. (2012). Modulation of L-α-lysophosphatidylinositol/GPR55 mitogen-activated protein kinase (MAPK) signaling by cannabinoids. J. Biol. Chem. 287, 91–104.
Cascio, M.G., Zamberletti, E., Marini, P., Parolaro, D., and Pertwee, R.G. (2014). The phytocannabinoid, Δ(9) -tetrahydrocannabivarin, can act through 5-HT1A receptors to produce anti-psychotic effects. Br. J. Pharmacol.
Han, Z., Fang, Q., Wang, Z., Li, X., Li, N., Chang, X., Pan, J., Tang, H., and Wang, R. (2014). Antinociceptive effects of central administration of the endogenous cannabinoid receptor type 1 agonist VDPVNFKLLSH-OH [(m)VD-Hemopressin(α)], an N-terminally extended Hemopressin peptide. J. Pharmacol. Exp. Ther. 348, 316–323.
Hill, A.J., Jones, N.A., Smith, I., Hill, C.L., Williams, C.M., Stephens, G.J., and Whalley, B.J. (2014). Voltage-gated sodium (NaV) channel blockade by plant cannabinoids does not confer anticonvulsant effects per se. Neurosci. Lett. 566, 269–274.
Husni, A.S., McCurdy, C.R., Radwan, M.M., Ahmed, S.A., Slade, D., Ross, S.A., ElSohly, M.A., and Cutler, S.J. (2014). Evaluation of Phytocannabinoids from High Potency Cannabis sativa using In Vitro Bioassays to Determine Structure-Activity Relationships for cannabinoid Receptor 1 and cannabinoid Receptor 2. Med. Chem. Res. Int. J. Rapid Commun. Des. Mech. Action Biol. Act. Agents 23, 4295–4300.
McPartland, J.M., Guy, G.W., and Di Marzo, V. (2014). Care and feeding of the endocannabinoid system: a systematic review of potential clinical interventions that upregulate the endocannabinoid system. PloS One 9, e89566.
Overton, H.A., Babbs, A.J., Doel, S.M., Fyfe, M.C.T., Gardner, L.S., Griffin, G., Jackson, H.C., Procter, M.J., Rasamison, C.M., Tang-Christensen, M., et al. (2006). Deorphanization of a G protein-coupled receptor for oleoylethanolamide and its use in the discovery of small-molecule hypophagic agents. Cell Metab. 3, 167–175.
Pertwee, R.G., Howlett, A.C., Abood, M.E., Alexander, S.P.H., Di Marzo, V., Elphick, M.R., Greasley, P.J., Hansen, H.S., Kunos, G., Mackie, K., et al. (2010). International Union of Basic and Clinical Pharmacology. LXXIX. cannabinoid receptors and their ligands: beyond CB₁ and CB₂. Pharmacol. Rev. 62, 588–631.
Russo, E.B., Burnett, A., Hall, B., and Parker, K.K. (2005). Agonistic properties of cannabidiol at 5-HT1A receptors. Neurochem. Res. 30, 1037–1043.