
Latest Blog Logo Post
11 years 7 months ago
Etharums ser quidem rerum facilis dolores nemis omnis fugats vitaes nemo minima rerums unsers sadips amets.

Lorem ipsum dolor sit amet, consectetur adipiscing elit. Donec nec eros eget nisl fringilla commodo. Maecenas ornare, augue ut ultricies tristique, enim lectus pretium quam, quis bibendum metus tellus sed magna. Donec eu dolor Pellentesque pellentesque tempor tellus eget…

11 years 7 months ago

11 years 7 months ago

11 years 7 months ago
GH MEDICAL White Paper Portuguese PDF
GH MEDICAL White Paper German PDF
GH MEDICAL White Paper Dutch PDF
Like with most medication, the therapeutic use of cannabis or cannabinoids can elicit adverse effects such as tachycardia, dry mouth, blurred vision, vertigo, low blood pressure, panic attack, red eyes or itchy skin.
Similar to its therapeutic effects, the adverse effects related to cannabis use are mostly due to THC and its effect on the CB1 receptor.
Although these effects are generally innocuous and transient, they can be upsetting for uninformed or inexperienced patients.
Here we discuss known adverse effects of cannabis / THC intoxication and, where possible, which countermeasures can be taken.
Unless otherwise stated, the information in this section refers to the “Adverse Effects” section in “Information for Health Care Professionals: Cannabis and the cannabinoids”.
Toxicological effects
Although cannabinoids can exert very strong physical and psychological effects, they are actually quite mild from a toxicological point of view. cannabinoids can be cytotoxic to certain types of cancer cells (for instance see: Gustafsson et al., 2009; Powles et al., 2005) but not under normal physiological conditions.
Similarly, cannabinoids have little or no mutagenic potential under normal conditions and are therefore unlikely to cause cancer for instance.
This results in an unusually high Margin Of Exposure/MOE: the ratio of the lowest dose required for the desired (therapeutic) effect and the dose that elicits adverse or potentially lethal effects. Many therapeutic drugs, but also recreational drugs such as alcohol or nicotine have MOEs <10, meaning that it is relatively easy to overdose. Opiates, cocaine, amphetamines, ecstasy and benzodiazepines typically have MOEs >100. Cannabis on the other hand has an MOE >10.000 making it a very safe drug (Lachenmeier and Rehm, 2015).
Please note that although cannabis and cannabinoids are relatively innocuous in themselves, smoking cannabis, like tobacco produces compounds that are cytotoxic and mutagenic (Moir et al., 2008). This is relevant as the majority of therapeutic users prefers smoked/inhaled cannabis (Sexton et al., 2016).
Acute physical effects
· Tachycardia: The most common side effect of cannabis (read THC) consumption is tachycardia. This is transient and dose-related and usually innocuous in healthy young users. However, patients, especially those with a heart condition, should be cautious and contact a physician when in doubt.
· Dry mouth: Cannabis consumption can lead to a dry mouth. This is due to activation of CB1 and CB2 receptors in the submandibular gland (Prestifilippo et al., 2006) and is generally considered innocuous.
· Blurred vision: Cannabis consumption can lead to blurred vision. This is probably a result of THC lowering intra-ocular pressure (which is the basis of the therapeutic use of cannabis against glaucoma). Blurred vision of cannabis use is transient en in itself innocuous.
· Nausea/dizziness: Nausea and dizziness are among the most reported side effects of cannabis (read THC) consumption. The effects are dose-dependent, transient and generally innocuous.
· Low blood pressure: Cannabis consumption can lead to peripheral vasodilation and subsequent low blood pressure. These effects are dose-dependent, transient and generally innocuous.
· Red eyes: Cannabis-induced peripheral vasodilation can cause ‘blood-shot’ or red eyes. This effect is transient and innocuous.
· Itchy skin: Although cannabis is often used to treat itchy skin or similar disorders, itchy skin can also be a side-effect of cannabis use. Itchy skin is transient and harmless.
· Hunger / low blood sugar: Cannabis use is associated with lowered blood sugar levels (Penner et al., 2013) and increased appetite. For this reason cannabis is used to treat Diabetes and anorexia-like symptoms but it can also be experienced and adverse effect. Cannabis-induced low blood sugar is transient and generally harmless.
· Hypothermia: THC in cannabis can produce hypothermia via the CB1 receptor. This effect is transient and generally harmless. Please note that while CBD can revert several effects of THC, it tends to exacerbate THC-induced hypothermia (Taffe et al., 2015)
· Reduced motor control: THC in cannabis can reduce motor control and locomotion. This effect is transient and generally harmless. Please note that while CBD can revert several effects of THC, it tends to exacerbate THC-induced hypolocomotion (Taffe et al., 2015).
Acute psychological effects
· Short-term memory loss: One of the most notable side effects of cannabis use is short-term memory loss. Although this is beneficial for instance for the treatment of posttraumatic stress disorders or for generalized stress relief, it can also be experienced as an adverse effect. Memory loss is induced via THC and CB1 and is transient and innocuous.
· Loss of concentration: As a direct consequence of reduced short-term memory function, cannabis use can also lead to loss of focus or concentration. These effects are mediated via THC and CB1 and are transient in nature.
· Panic attack/paranoia: Although cannabis can be used to treat anxiety or stress, its use can also elicit a panic attack or paranoia. These panic attacks are likely a result of the acute effects mentioned above and are mediated by THC. Cannabis-induced anxiety or paranoia is transient and in itself harmless.
Countermeasures
Acute adverse effects of cannabis are transient and are usually easily counteracted with activity, controlled breathing and sugar.
As most adverse effects directly or indirectly result from low blood pressure or low blood sugar, light activity such as walking and consuming sugary food or drinks are often sufficient to abolish these adverse effects in minutes.
Similarly, deep belly breathing can often revert adverse effects such as panic or anxiety in seconds or minutes.
In addition, many effects of THC can be counteracted by CBD although it should be noted that CBD can paradoxically increase the effects of THC by inhibiting its degradation via inhibition of Cytochrome P450 enzymes.
Long-term effects and contraindications
· Carcinogenesis: cannabinoids themselves are not carcinogenic or mutagenic. However, smoking is a popular route of cannabinoid administration and smoking cannabis, like smoking tobacco produces carcinogenic compounds. Interestingly, compared to smoking tobacco, long-term smoking of cannabis actually reduces the risk of cancer in squamous cells lining the respiratory tract in head and neck by approximately 50% (Liang et al., 2009). Vaping or ingesting cannabinoids is likely to greatly reduce the risk of carcinogenesis.
· Respiratory tract: cannabinoids themselves do not negatively influence the respiratory tract. However, smoking is a popular route of cannabinoid administration and smoking cannabis, like smoking tobacco produces pathogenic/carcinogenic compounds. In addition, cannabis smoke tends to be inhaled longer and deeper, resulting in increased tar and carbon monoxide levels compared to smoking tobacco. As a result, biopsies from chronic cannabis smokers often show histopathological changes such as basal cell hyperplasia, stratification, goblet cell hyperplasia, inflammation, basement membrane thickening and squamous cell metaplasia. Vaping or ingesting cannabis is likely to greatly reduce the risk of respiratory tract disease.
· Immune system: The endocannabinoid system is prominently involved in immune regulation. As a consequence, cannabinoids have great regulatory potential in the immune system. Tight regulation of the immune system is crucial to healthy life: an overactive immune system can evoke auto-immune disorders whereas a weak immune system leaves the body prone to infection. THC appears to be mainly anti-inflammatory at low nanomolar concentrations but pro-inflammatory at low micromolar concentrations (Berdyshev et al., 1997). Cannabis use is mostly associated with immunosuppression/anti-inflammatory action, similar to most anti-inflammatory drugs. It is important to realize that chronic cannabis use therefore roughly equates to chronic immunosuppression, which in turn could negatively impact general health.
· Reproductive system:
o Behavior: Cannabis use can influence many types of behavior, including sexual behavior in a dose-dependent way. Both men and women tend to respond positively to occasional low or moderate doses of cannabis with increased sensitivity to touch, increased desire and increased sexual activity. Higher doses or frequency of use can have the opposite effect and inhibit sexual motivation or erectile function for instance.
o Male reproduction: The endocannabinoid system is not just present in the brain (affecting behavior) but in most tissues including reproductive tissues and it can thus influence many aspects of reproductive physiology. An in vitro study showed that THC dose-dependently reduces sperm motility and inhibits the acrosome reaction (Whan et al., 2006). It should be noted though that THC primarily affects motility in ‘poor swimming sperm’. Motility in ‘fast swimming sperm’ is only affected by very high THC concentrations (4.8 μM or ±1500 μg/l plasma), which are not likely to reached after therapeutic or even heavy recreational use of cannabis. Still, cannabis/THC can negatively influence fertility, especially in males that are on the border of infertility.
o Female reproduction: Animal and in vitro studies have shown that cannabis/THC can negatively affect ovulation and successful pregnancy (Kostellow et al., 1980; Yao et al., 2018) but only at very high concentrations, unlikely to be attained in humans. In fact, a systematic review in humans failed to find a negative impact of cannabis use during pregnancy on fetal or child development (Zhang et al., 2017).
· Cardiovascular system: As discussed above, tachycardia and peripheral vasodilation are consistent acute side effects of cannabis use. In healthy young people this is harmless but in elderly people, medical cannabis users or patients with cardiovascular problems this could be relevant. To put things in perspective, smoking cannabis elevates the risk of myocardial infection at population level, but less so then eating a heavy meal. At the individual level, smoking cannabis elevates the risk of heart attack less than exposure to traffic, alcohol, coffee, air pollution, negative emotions, anger, a heavy meal, positive emotions, sex or cocaine use (Nawrot et al., 2011).
· Gastrointestinal system: One adverse effect that is occasionally observed with chronic heavy cannabis use is ‘cannabis hyperemesis syndrome’. Until recently the only remedy was abstinence from cannabis use and taking hot showers. In line with the latter observation, the TRPV1 receptor is now implicated in cannabis hyperemesis syndrome and treatment with topical capsaicin (TRPV1 agonist) appears effective (Dezieck et al., 2017; Moon et al., 2018). Other reported treatments for hyperemesis syndrome are haloperidol (often used as anti-psychotic and anti-emetic) and propranolol (often used against high blood pressure and anxiety) (Jones and Abernathy, 2016; Richards and Dutczak, 2017).
· Central nervous system
o Cognition: Short-term memory loss is a well-known acute effect of cannabis use. Whether chronic cannabis use has a long-term effect on cognition remains controversial and requires more research.
o Motor control: Although cannabis use acutely and negatively affects motor control there is no indication that cannabis can negatively affect motor control on a longer term.
o Mental disorders: Several mental disorders such as depression, anxiety, bipolar disorder, psychosis and schizophrenia have been associated with cannabis use but a causal link was never shown. In fact, several mental disorders are now linked to mutations in the genes underlying the endocannabinoid system / ECS variation, suggesting cannabinoids may be used to treat these very disorders. Regardless, caution is advised for the use of cannabinoids in patients with a history of mental disease.
· Addiction: Like other psychoactive drugs, cannabis (read THC) has the potential to develop psychological dependence and to a degree physical dependence. Whether cannabis or cannabinoid use leads to dependence relies on several factors:
o Genetic passport: every individual has a unique genetic passport, which determines which diseases we are likely to get. Many diseases are now beginning to be recognized as ECS deficiencies, suggesting these can be treated with cannabinoid supplements. Abstinence from cannabinoids is likely to affect people with an ECS deficiency harder than people with a fully functional ECS.
o Route of application: Addiction or dependence is mostly attributed to the smoking of cannabis, especially when mixed with tobacco. Oral or sublingual application of cannabinoids is far less likely to cause dependence. Routes of application that do not cause psychoactive effects (for instance topical application to the skin) are not considered addictive.
o Psychoactivity of cannabinoids: Cannabis / THC is considered addictive mostly because of its psychoactive properties. cannabinoids such as CBD are not psychoactive and are considered to have no abuse potential. In fact, CBD can be used to suppress or counteract the addictive potential of cannabis / THC (Crippa et al., 2013), alcohol (Viudez-Martínez et al., 2017) or tobacco (Morgan et al., 2013).
References:
Berdyshev, E.V., Boichot, E., Germain, N., Allain, N., Anger, J.P., and Lagente, V. (1997). Influence of fatty acid ethanolamides and delta9-tetrahydrocannabinol on cytokine and arachidonate release by mononuclear cells. Eur. J. Pharmacol. 330, 231–240.
Crippa, J. a. S., Hallak, J.E.C., Machado-de-Sousa, J.P., Queiroz, R.H.C., Bergamaschi, M., Chagas, M.H.N., and Zuardi, A.W. (2013). Cannabidiol for the treatment of cannabis withdrawal syndrome: a case report. J. Clin. Pharm. Ther. 38, 162–164.
Dezieck, L., Hafez, Z., Conicella, A., Blohm, E., O’Connor, M.J., Schwarz, E.S., and Mullins, M.E. (2017). Resolution of cannabis hyperemesis syndrome with topical capsaicin in the emergency department: a case series. Clin. Toxicol. Phila. Pa 1–6.
Gustafsson, S.B., Lindgren, T., Jonsson, M., and Jacobsson, S.O.P. (2009). cannabinoid receptor-independent cytotoxic effects of cannabinoids in human colorectal carcinoma cells: synergism with 5-fluorouracil. cancer Chemother. Pharmacol. 63, 691–701.
Jones, J.L., and Abernathy, K.E. (2016). Successful Treatment of Suspected cannabinoid Hyperemesis Syndrome Using Haloperidol in the Outpatient Setting. Case Rep. Psychiatry 2016, 3614053.
Kostellow, A.B., Ziegler, D., Kunar, J., Fujimoto, G.I., and Morrill, G.A. (1980). Effect of cannabinoids on estrous cycle, ovulation and reproductive capacity of female A/J mice. Pharmacology 21, 68–75.
Lachenmeier, D.W., and Rehm, J. (2015). Comparative risk assessment of alcohol, tobacco, cannabis and other illicit drugs using the margin of exposure approach. Sci. Rep. 5.
Liang, C., McClean, M.D., Marsit, C., Christensen, B., Peters, E., Nelson, H.H., and Kelsey, K.T. (2009). A population-based case-control study of marijuana use and head and neck squamous cell carcinoma. cancer Prev. Res. Phila. Pa 2, 759–768.
Moir, D., Rickert, W.S., Levasseur, G., Larose, Y., Maertens, R., White, P., and Desjardins, S. (2008). A comparison of mainstream and sidestream marijuana and tobacco cigarette smoke produced under two machine smoking conditions. Chem. Res. Toxicol. 21, 494–502.
Moon, A.M., Buckley, S.A., and Mark, N.M. (2018). Successful Treatment of cannabinoid Hyperemesis Syndrome with Topical Capsaicin. ACG Case Rep. J. 5, e3.
Morgan, C.J.A., Das, R.K., Joye, A., Curran, H.V., and Kamboj, S.K. (2013). Cannabidiol reduces cigarette consumption in tobacco smokers: preliminary findings. Addict. Behav. 38, 2433–2436.
Nawrot, T.S., Perez, L., Künzli, N., Munters, E., and Nemery, B. (2011). Public health importance of triggers of myocardial infarction: a comparative risk assessment. Lancet Lond. Engl. 377, 732–740.
Penner, E.A., Buettner, H., and Mittleman, M.A. (2013). The impact of marijuana use on glucose, insulin, and insulin resistance among US adults. Am. J. Med. 126, 583–589.
Powles, T., te Poele, R., Shamash, J., Chaplin, T., Propper, D., Joel, S., Oliver, T., and Liu, W.M. (2005). Cannabis-induced cytotoxicity in leukemic cell lines: the role of the cannabinoid receptors and the MAPK pathway. Blood 105, 1214–1221.
Prestifilippo, J.P., Fernández-Solari, J., de la Cal, C., Iribarne, M., Suburo, A.M., Rettori, V., McCann, S.M., and Elverdin, J.C. (2006). Inhibition of salivary secretion by activation of cannabinoid receptors. Exp. Biol. Med. Maywood NJ 231, 1421–1429.
Richards, J.R., and Dutczak, O. (2017). Propranolol Treatment of cannabinoid Hyperemesis Syndrome: A Case Report. J. Clin. Psychopharmacol.
Sexton, M., Cuttler, C., Finnell, J.S., and Mischley, L.K. (2016). A Cross-Sectional Survey of Medical Cannabis Users: Patterns of Use and Perceived Efficacy. Cannabis cannabinoid Res. 1, 131–138.
Taffe, M.A., Creehan, K.M., and Vandewater, S.A. (2015). Cannabidiol fails to reverse hypothermia or locomotor suppression induced by Δ(9) -tetrahydrocannabinol in Sprague-Dawley rats. Br. J. Pharmacol. 172, 1783–1791.
Viudez-Martínez, A., García-Gutiérrez, M.S., Navarrón, C.M., Morales-Calero, M.I., Navarrete, F., Torres-Suárez, A.I., and Manzanares, J. (2017). Cannabidiol reduces ethanol consumption, motivation and relapse in mice. Addict. Biol.
Whan, L.B., West, M.C.L., McClure, N., and Lewis, S.E.M. (2006). Effects of delta-9-tetrahydrocannabinol, the primary psychoactive cannabinoid in marijuana, on human sperm function in vitro. Fertil. Steril. 85, 653–660.
Yao, J.L., He, Q.Z., Liu, M., Chang, X.W., Wu, J.T., Duan, T., and Wang, K. (2018). Effects of Δ(9)-tetrahydrocannabinol (THC) on human amniotic epithelial cell proliferation and migration. Toxicology 394, 19–26.
Zhang, A., Marshall, R., and Kelsberg, G. (2017). Clinical Inquiry: What effects--if any--does marijuana use during pregnancy have on the fetus or child? J. Fam. Pract. 66, 462–466.
Is there a difference between natural and synthetic cannabinoids?
In principle exact copies of naturally occurring cannabinoids can be synthesized. Therefore, synthetic cannabinoids are not necessarily different from natural cannabinoids. However, in practice synthetic cannabinoids are produced to be more specific and more powerful than natural cannabinoids, which can have a dramatic effect on their safety profile.
Entourage effect
Although exact copies of natural cannabinoids can be synthesized, natural cannabinoids from plants or extracts are flanked by numerous other compounds such as other cannabinoids or terpenes whereas synthetic cannabinoids usually are not.
Like the main ingredient / the cannabinoid of interest, these other compounds also bind one or more cannabinoid receptors and will therefore alter the effect that the cannabinoid of interest has.
Although it is not exactly known how this so called ‘entourage effect’ works, it is known that it consistently improves the therapeutic potential of cannabinoids (Gallily et al., 2015; Russo, 2011; Russo and Guy, 2006).
Specificity
Natural cannabinoids typically bind to more than one receptor and therefore exert more than one effect. While this ‘promiscuity’ of natural cannabinoids may be very useful in normal physiology this can frustrate research attempting to elucidate the exact mechanism behind a disease or a therapy. Therefore, for research purposes, synthetic compounds / cannabinoids that are highly specific for a particular receptor can be very useful. For instance mapping the distribution of CB1 receptors can only be done faithfully when the used probe is highly specific for CB1 and does not cross-react with any other receptor in the body (Ceccarini et al., 2015).
Efficacy
Natural cannabinoids typically are gentle modulators of receptor activity that have a moderate affinity for their receptor that is short-lasting and can be washed away by other compounds / modulators. Synthetic cannabinoids are often designed to have a high affinity for their receptor and have a lasting effect.
THC for example is a partial agonist for CB1. If CB1 were a car engine, THC would put it in first gear. It’s synthetic analogue, Win55212-2 is a full agonist, which would put CB1 in high gear with a brick on the accelerator. SR141716 is a synthetic CB1 antagonist, which would slam on the break and bring the CB1 engine to a full stop. CBD does not seem to bind directly to the activation site of CB1 but to other parts of the receptor, thereby modulating or 'steering' the function of CB1 (see figure below).
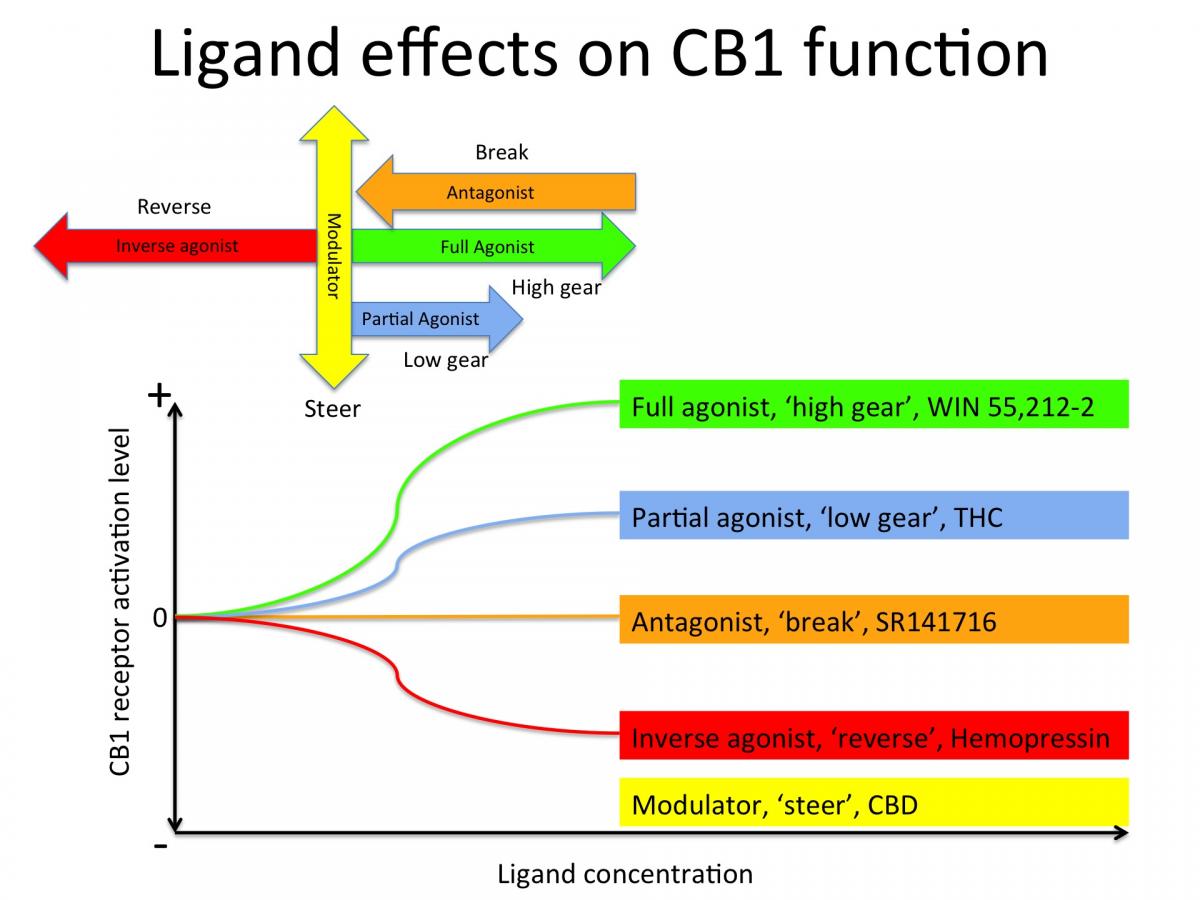
Safety
Although synthetic cannabinoids can be very useful in basic research to elucidate molecular mechanisms in physiological processes, they can be dangerous if not lethal in clinical use / humans. For instance, rimonabant / SR141716 was introduced as an anti-obesity drug but subsequently pulled of the market for its potential to trigger serious psychiatric side effects or even suicide. With the advent of both recreational and medicinal cannabis the market is increasingly flooded with synthetic cannabinoids. This is at least partially due to the fact that synthetic compounds can be patented and therefore more profitable than natural cannabinoids. As a result serious injuries and even fatalities due to synthetic cannabinoid use are now reported almost on a daily basis (Adedinsewo et al., 2016; van Amsterdam et al., 2015; Babi et al., 2017; Barceló et al., 2017; Buyukbese Sarsu, 2016; Cha et al., 2015; Clark et al., 2015; Degirmenci et al., 2015; Demir et al., 2017; Efe et al., 2017; Ezaki et al., 2016; FuNADA and Takebayashi-Ohsawa, 2017; Karass et al., 2017; Kırgız and Kaldırım, 2017; Kusano et al., 2017; Mansoor et al., 2017; McIlroy et al., 2016; Moeller et al., 2017; Monte et al., 2017; Öcal et al., 2016; Ozturk et al., 2017; Raheemullah and Laurence, 2016; Samra et al., 2017; Shanks and Behonick, 2016; Waugh et al., 2016; Zarifi and Vyas, 2017).
Natural cannabis / cannabinoids has been used around the world for over 6.000 years without a single reported fatality and are thus far, far safer than synthetic cannabinoids.
References:
Adedinsewo, D.A., Odewole, O., and Todd, T. (2016). Acute Rhabdomyolysis Following Synthetic cannabinoid Ingestion. North Am. J. Med. Sci. 8, 256–258.
van Amsterdam, J., Brunt, T., and van den Brink, W. (2015). The adverse health effects of synthetic cannabinoids with emphasis on psychosis-like effects. J. Psychopharmacol. Oxf. Engl.
Babi, M.-A., Robinson, C.P., and Maciel, C.B. (2017). A spicy status: Synthetic cannabinoid (spice) use and new-onset refractory status epilepticus-A case report and review of the literature. SAGE Open Med. Case Rep. 5, 2050313X17745206.
Barceló, B., Pichini, S., López-Corominas, V., Gomila, I., Yates, C., Busardò, F.P., and Pellegrini, M. (2017). Acute intoxication caused by synthetic cannabinoids 5F-ADB and MMB-2201: A case series. Forensic Sci. Int. 273, e10–e14.
Buyukbese Sarsu, S. (2016). Unusual side effect of cannabis use: acute abdomen due to duodenal perforation. Int. J. Emerg. Med. 9, 18.
Ceccarini, J., Kuepper, R., Kemels, D., van Os, J., Henquet, C., and Van Laere, K. (2015). [18F]MK-9470 PET measurement of cannabinoid CB1 receptor availability in chronic cannabis users. Addict. Biol. 20, 357–367.
Cha, H.J., Seong, Y.-H., Song, M.-J., Jeong, H.-S., Shin, J., Yun, J., Han, K., Kim, Y.-H., Kang, H., and Kim, H.S. (2015). Neurotoxicity of Synthetic cannabinoids JWH-081 and JWH-210. Biomol. Ther. 23, 597–603.
Clark, B.C., Georgekutty, J., and Berul, C.I. (2015). Myocardial Ischemia Secondary to Synthetic cannabinoid (K2) Use in Pediatric Patients. J. Pediatr.
Degirmenci, Y., Kececi, H., and Olmez, N. (2015). A case of ischemic stroke after bonzai: syntetic cannabinoid from Turkey. Neurol. Sci. Off. J. Ital. Neurol. Soc. Ital. Soc. Clin. Neurophysiol.
Demir, T., Onan, H.B., Salkin, F.O., and Bicakci, S. (2017). Distal internal carotid artery dissection after consumption of synthetic cannabinoid “Bonzai.” Neurol. Neurochir. Pol.
Efe, T.H., Felekoglu, M.A., Çimen, T., and Doğan, M. (2017). Atrial fibrillation following synthetic cannabinoid abuse. Turk Kardiyol. Dernegi Arsivi Turk Kardiyol. Derneginin Yayin Organidir 45, 362–364.
Ezaki, J., Ro, A., Hasegawa, M., and Kibayashi, K. (2016). Fatal overdose from synthetic cannabinoids and cathinones in Japan: demographics and autopsy findings. Am. J. Drug Alcohol Abuse 1–10.
FuNADA, M., and Takebayashi-Ohsawa, M. (2017). Synthetic cannabinoid AM2201 induces seizures: Involvement of cannabinoid CB1 receptors and glutamatergic transmission. Toxicol. Appl. Pharmacol. 338, 1–8.
Gallily, R., Yekhtin, Z., and Hanuš, L.O. (2015). Overcoming the Bell-Shaped Dose-Response of Cannabidiol by Using Cannabis Extract Enriched in Cannabidiol. Pharmacol. Amp Pharm. 06, 75.
Karass, M., Chugh, S., Andries, G., Mamorska-Dyga, A., Nelson, J.C., and Chander, P.N. (2017). Thrombotic microangiopathy associated with synthetic cannabinoid receptor agonists. Stem Cell Investig. 4, 43.
Kırgız, A., and Kaldırım, H. (2017). Bilateral multiple exudative retinal detachments and macular edema in a patient diagnosed with synthetic cannabinoid (Bonzai) intoxication. Int. Ophthalmol.
Kusano, M., Zaitsua, K., Taki, K., Hisatsune, K., Nakajima, J., Moriyasu, T., Asano, T., Hayashi, Y., Tsuchihashi, H., and Ishii, A. (2017). Fatal Intoxication by 5F-ADB and Diphenidine: Detection, Quantification, and Investigation of their Main Metabolic Pathways in Human by LC/MS/MS and LC/Q-TOFMS. Drug Test. Anal.
Mansoor, K., Zawodniak, A., Nadasdy, T., and Khitan, Z.J. (2017). Bilateral renal cortical necrosis associated with smoking synthetic cannabinoids. World J. Clin. Cases 5, 234–237.
McIlroy, G., Ford, L., and Khan, J.M. (2016). Acute myocardial infarction, associated with the use of a synthetic adamantyl-cannabinoid: a case report. BMC Pharmacol. Toxicol. 17, 2.
Moeller, S., Lücke, C., Struffert, T., Schwarze, B., Gerner, S.T., Schwab, S., Köhrmann, M., Machold, K., Philipsen, A., and Müller, H.H. (2017). Ischemic stroke associated with the use of a synthetic cannabinoid (spice). Asian J. Psychiatry 25, 127–130.
Monte, A.A., Calello, D.P., Gerona, R.R., Hamad, E., Campleman, S.L., Brent, J., Wax, P., Carlson, R.G., and ACMT Toxicology Investigators Consortium (ToxIC) (2017). Characteristics and Treatment of Patients with Clinical Illness Due to Synthetic cannabinoid Inhalation Reported by Medical Toxicologists: A ToxIC Database Study. J. Med. Toxicol. Off. J. Am. Coll. Med. Toxicol.
Öcal, N., Doğan, D., Çiçek, A.F., Yücel, O., and Tozkoparan, E. (2016). Acute Eosinophilic Pneumonia with Respiratory Failure Induced by Synthetic cannabinoid Inhalation. Balk. Med. J. 33, 688–690.
Ozturk, H.M., Erdogan, M., Alsancak, Y., Yarlioglues, M., Duran, M., Boztas, M.H., Murat, S.N., and Ozturk, S. (2017). Electrocardiographic alterations in patients consuming synthetic cannabinoids. J. Psychopharmacol. Oxf. Engl. 269881117736918.
Raheemullah, A., and Laurence, T.N. (2016). Repeated Thrombosis After Synthetic cannabinoid Use. J. Emerg. Med.
Russo, E.B. (2011). Taming THC: potential cannabis synergy and phytocannabinoid-terpenoid entourage effects. Br. J. Pharmacol. 163, 1344–1364.
Russo, E., and Guy, G.W. (2006). A tale of two cannabinoids: the therapeutic rationale for combining tetrahydrocannabinol and cannabidiol. Med. Hypotheses 66, 234–246.
Samra, K., Boon, I.S., Packer, G., and Jacob, S. (2017). Lethal high: acute disseminated encephalomyelitis (ADEM) triggered by toxic effect of synthetic cannabinoid black mamba. BMJ Case Rep. 2017.
Shanks, K.G., and Behonick, G.S. (2016). Death after use of the synthetic cannabinoid 5F-AMB. Forensic Sci. Int.
Waugh, J., Najafi, J., Hawkins, L., Hill, S.L., Eddleston, M., Vale, J.A., Thompson, J.P., and Thomas, S.H.L. (2016). Epidemiology and clinical features of toxicity following recreational use of synthetic cannabinoid receptor agonists: a report from the United Kingdom national poisons information service. Clin. Toxicol. Phila. Pa 1–7.
Zarifi, C., and Vyas, S. (2017). Spice-y Kidney Failure: A Case Report and Systematic Review of Acute Kidney Injury Attributable to the Use of Synthetic Cannabis. Perm. J. 21.